GammaCore Treats Migraine and Cluster Headaches
Migraine is a prevalent, disabling, genetically inherited headache syndrome present in 12% of the world’s population. Most persons prefer to take oral medications for acute treatment and others will use subcutaneous treatment for Migraine with CGRP modulators, like Aimovig.
Cluster headaches are the worst headaches of all and are called “suicide” headaches. Cluster headache responds to fast acting triptans like SC injectable sumatriptan and SC Emgality for prevention. The age-adjusted incidence of Cluster headache is 15.6 per 100,000 person-years for males and 4.0 per 100,000 person-years for females.
There is a need for a new way to treat Migraine and Cluster headaches, especially in persons who don’t tolerate usual treatment.
Both Migraine and Cluster headache patients commonly go to the Emergency Room for help.
This is an article by Britt Talley Daniel MD, member of the American Academy of Neurology, the American Headache Society, migraine textbook author and blogger.
Read my Mini Book on Migraine Here.
Emergency Room
GammaCore is a new medical device approved for acute and preventive treatment of Migraine and Cluster headache. GammaCore is an electric stimulator of the vagus nerve in the neck which has few side effects and is helpful for persons who have failed or do not like to take oral or injectable medication. GammaCore limits the possible development of medication overuse headache.
GammaCore nVNS (vagus nerve stimulator) provides relief for more than 50% of attacks for acute treatment of Migraine headache. GammaCore nVNS is “safe and effective.” Some patients felt relief in 30 minutes. Almost half of treated patients had little to almost no pain within 2 hours.
The majority of patients who were pain-free at 2 hours remained pain-free for 48 hours. GammaCore is effective for patients who have failed traditional oral or injectable medications such as triptans, caffeine, or NSAIDS.
GammaCore has also been approved for treating Cluster Headache.
Related Questions
1. What are the side effects of using GammaCore?
GammaCore treated patients did not have any serious treatment related side effects. Most of the reported side effects were mild, only occurring during the use of the device, and went away after each treatment. The most common side effects were discomfort and redness at the application site, dizziness, and a tingling feeling where the device was applied in the neck.
GammaCore has been shown to be safe and effective across a range of migraine populations in both the United States and Europe. Different treatment strategies have been evaluated showing good clinical, safety, and quality of life outcomes at various treatment study times.
2. How does GammaCore (nVNS) work?
GammaCore is a vagus nerve stimulator. The vagus nerve is one of the largest nerves in the parasympathetic division of the autonomic nervous system. GammaCore activates the vagus nerve by mild electrical stimulation and thereby decreases pain perception. GammaCore therapy is not indicated for children, pregnant women, or patients with active implantable medical devices.
It is also not indicated for patients with carotid atherosclerosis, hypertension, bradycardia/tachycardia, or those with a metallic device. It shouldn’t be used in patients with a stent, bone plate, or bone screw near the neck. It also shouldn't be used at the same time as the use of a mobile phone or other portable electronic device.
GammaCore Device
3. What is the function of the vagus nerve?
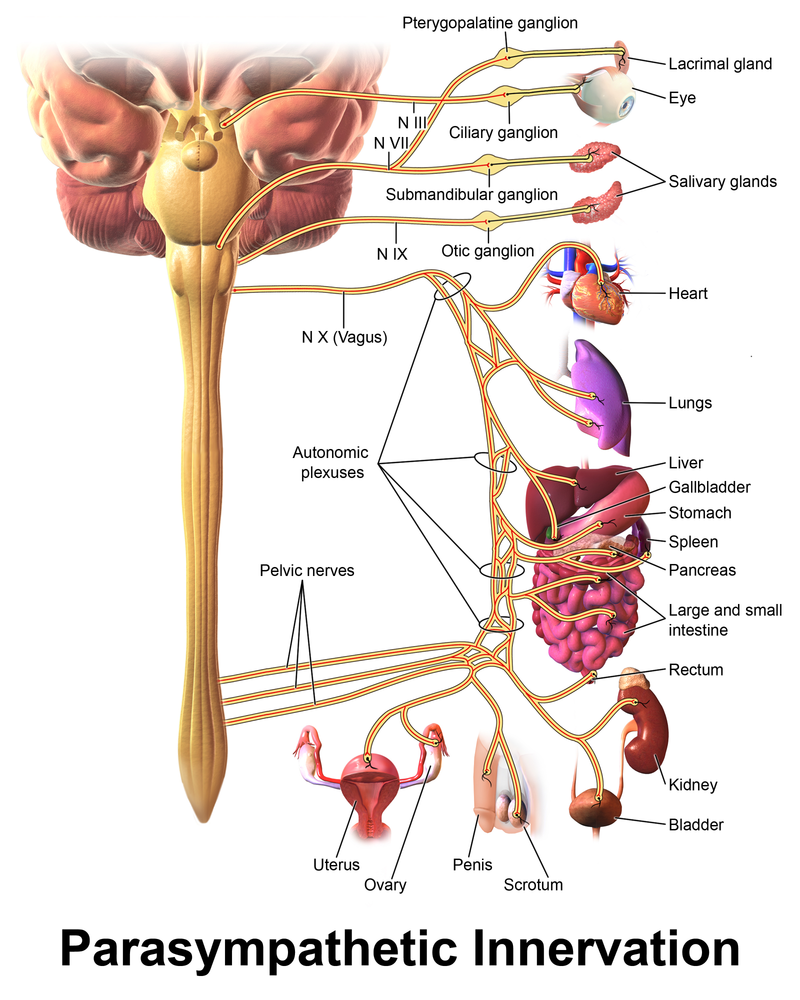
The vagus nerve connects the medulla of the brain stem with various target organs in the body. One of the most well know functions of the vagus nerve is to cause the cells in the lining of the stomach to secrete acid to aid digestion. The vagus nerve helps regulate pain. The vagus nerve shuts off inflammation in the body and migraine is an inflammatory medical condition. Vagus nerve stimulators are also used to treat depression and epilepsy.
The vagus nerve has the longest course of all the cranial nerves, extending from the medulla to the abdomen. Its name is derived from the Latin “vagary” which means “wandering.” The vagus nerve originates from the medulla of the brainstem to neurons in the stomach, the upper and lower bowel, the bladder, and all the sexual organs.
Vagus Nerve
4. How does Electrical treatment work?
GammaCore is a new treatment option for Migraine and Cluster headache. It is a new electrical vagus nerve stimulator which provides drug free treatment.
GammaCore, produces a proprietary low-voltage electrical signal comprising a 5 kHz sine wave burst lasting for 1 millisecond (5 sine waves, each lasting 200 microseconds). Such bursts repeated once every 40 milliseconds (25 Hz) generating a 24 V peak voltage and 60 mA peak output current.
These electrical stimulations active the Vagus nerve and decrease pain perception.
The device is placed vertically below the chin and stimulations are made until the patient notices contraction of the lower lip on the treated side, indicating location of stimulation over the vagus nerve.
5. What were the clinical results of using GammaCore for Migraine?
A prospective double-blind, sham-controlled studies of nVNS for the acute treatment of migraine was performed, (PRESTO), and the noninvasive neurostimulation for the prevention of chronic migraine (EVENT) trial were performed.
PRESTO included 243 patients with episodic migraine. More members receiving nVNS were pain free at 30 minutes (12.7%) than those receiving a sham treatment (4.2%; P = .01). There was also a greater percentage of the nVNS group who were pain free at 60 minutes (21% vs 10%, respectively; P= .02).
"The PRESTO data suggests that GammaCore was rapidly effective, well-tolerated, and practical for the acute treatment of episodic migraine," principal investigator, Cristina Tassorelli, MD, director of the Headache Science Center at the C. Mondino National Neurological Institute, Pavia, Italy, in a press announcement released in 2017.
In the EVENT study, 59 chronic patients with migraine for prevention treatment formed the study population (GammaCore, n=30 vs sham, n=29). Persistent prophylactic GammaCore use was associated with continued reductions in the number of headache days.
6. What is Migraine?
Migraine is a genetic condition that is inherited through your DNA. The genes that transmit migraine have been found and published in the medical literature. Migraine occurs in 25 % of women and 6 % of men. For women migraine is their most common chronic medical problem and more prevalent than diabetes, heart disease, or arthritis. Migraine is the fifth most common cause of disability worldwide in men and women and causes a large amount of clinical and economic burden.
Migraine causes more than a million ER visits every year. An attack of migraine according to the International Classification of Headache Disorders 3 lasts 4-72 hours and is called episodic migraine if it occurs less than 14 days per month, or chronic migraine if there are more than 15 headache days a month, 8 of which have migraine features.
7. What is the usual pharmaceutical treatment of Migraine?
Current acute therapy for migraine involves using NSAIDs, caffeine, DHE, one of the 7 triptans, one of 2 new CGRP receptor blockers, or Reyvow. All medications may have possible side effects and too much medication may lead to medication overuse headache.
8. What is Cluster headache?
Here’s the definition from the The International Classification of Headache Disorders-3. Cluster headache is:
A. At least 5 attacks fulfilling B-D below.
B. Severe unilateral orbital, supraorbital, and/or temporal pain lasting 15 to 180 min.
C. Attack is associated with at least one of the following signs on the side of pain:
1. Conjunctival injection
2. Lacrimation
3. Nasal congestion
4. Rhinorrhea
5. Forehead and facial sweating
6. Miosis
7. Ptosis
8. Eyelid edema
D. Frequency: from one every other day to eight per day
Clinical features
During the cluster headache in addition to pain the patient may experience symptoms on one side of the face around the eye, upper cheek, or temple. These symptoms may be:
drooping of the upper eyelid (ptosis)
8. What were the results of using GammaCore for Cluster Headache?
GammaCore provided significant relief for prevention and acute therapy for episodic Cluster Headache and has been approved for its use.
9. What are the usual pharmaceutical ways of treating Cluster Headache?
Cluster headache is usually treated acutely with subcutaneous injection of sumatriptan 6 mg or zomatriptan nasal spray 5 mg at onset. Preventive treatment has in the past been daily oral dosing with verapamil. Emgality, a CGRP receptor blocker has recently been approved for Cluster Headache.
GammaCore preventive treatment for Cluster Headache is 2 treatments, one in the morning and one at night, of 3 separate two-minute stimulations. GammaCore stimulations may be given on the same side of the neck or it may be switched to the other side as tolerated.
10. Summary
GammaCore electrical vagus nerve stimulation is a new option for treatment of Migraine and Cluster headache, which is especially helpful for patients not responsive to usual treatment options or for persons with medication overuse headache, which is common with migraineurs and rare but present in Cluster patients.
Check our my Big Book on Migraine, which also has an extensive discussion of the clinical description and treatment of Cluster Headache.
This site is owned and operated by Internet School LLC, a limited liability company headquartered in Dallas, Texas, USA. Internet School LLC is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.com. Although this site provides information about various medical conditions, the reader is directed to his own treating physician for medical treatment.
All the best.
Follow me at: www.doctormigraine.com, Pinterest, Amazon books, Podbean, and YouTube.
Britt Talley Daniel MD