Emgality (galcanezumab) For Migraine And Cluster Headaches
Migraines are very bad headaches for many persons. They are a common cause of disability and listed as one of the top causes of pain, along with childbirth, and a kidney stone. But cluster headaches are worse than migraines, although much more rare. So, considering this, it a very large advancement in medical treatment to have a single drug for both migraine and episodic cluster headache prevention.
Emgality is an antibody that blocks CGRP and has been approved by the FDA for prevention of both Migraine and Cluster Headache. Emgality reduces migraine attacks by 50-100% and cluster headaches by 50%. It is given by self administered subcutaneous injection once a month.
Does Emgality Help Migraine?
Emgality (galcanezumab) is a new Calcitonin Gene-Related Peptide (CGRP) drug for preventing migraine by acting as an antibody to interrupt the migraine cycle. The migraine process generates three neurochemicals, Neurokinan A, Substance P, and CGRP.
These neurochemicals are released by the ganglia of the trigeminal nerve and cerebral arteries. They inflame the trigeminal nerve, the cerebral arteries, and the thalamus. They also cause the cerebral arteries to dilate.
The thalamus is the “pain center” of the brain and only migraine inflames the thalamus like migraine does. Triptans, if taken early in the migraine process, 20-40 minutes after headache onset, block the release of these inflammatory neurochemicals and are the best drugs currently for acute therapy for migraine.
Research involving cannulating the jugular vein and sampling chemicals in the blood as it leaves the brain has revealed that these neurochemicals are released by the migraine process.
After migraine starts there is an increase in the 3 inflammatory neuropeptides, and they go to the liver and then out of the body to the toilet. Emgality blocks the activation of CGRP and is a new drug for migraine prevention. None of the older preventive drugs for migraine enter into the migraine process and work like this.
Emgality is the ONLY drug approved by the FDA for both Migraine and Cluster Headaches.
Read my Mini Book on Migraine
This is an article by Britt Talley Daniel MD, retired member of the American Academy of Neurology, the American Headache Society, migraine textbook author, and blogger.
The Migraine Timing Cycle
The picture above is the Migraine Timing Cycle and stage 2 shows the release of CGRP.
Read my article, “The Migraine Timing Cycle,” on my website, www.doctormigraine.com.
Related questions
How is Emgality administered? The first dose is 2 of the 120 mg subcutaneous injections, followed by one 120 mg injection once a month for migraine.
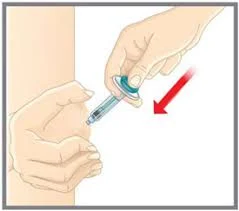
What kind of injector is used for Emgality? The injector for Emgality is shown below and is given in the anterior thigh or near the umbilicus.
Emgality subcutaneous injection
Are there any special precautions with using Emgality? Emgality should be stored in a refrigerator in its original carton to protect from light. The Emgality needle should be left at room temperature 30 minutes before injection. The thigh or abdomen should be cleaned with an alcohol sponge before injection.
Does Emgality interact with other drugs? Most modern drugs are made from the dirt or plants in the world and then are processed by drug companies and used to treat a specific illness.
For instance, penicillin is a mold that accidentally dropped on an agar plate Fleming had put on his window ledge. It was found to kill bacteria and later manufactured and sold to treat bacterial illnesses.
Penicillin as 10 different drug contraindications. This means that penicillin should not be used with those listed drugs. However, Emgality can be used with any drug and has no drug to drug contraindication.
Emgality has no contraindications with other drugs.
Emgality is made by DNA polymerization and then injected in the body. CGRP drugs are made by DNA polymerization, a lab process of spinning out strands of DNA. This is a completely novel method of drug production and safe regarding use with other existing drugs.
What are the side effects from taking Emgality for Migraine? Serious side effects are hypersensitivity reaction; common side effects are injection site reaction and muscle cramps or spasms.
Select Safety Information. Emgality may cause allergic reactions, such as itching, rash, hives, and trouble breathing. Allergic reactions can happen days after using Emgality.
Users are instructed to contact their healthcare provider or get emergency medical help right away if they experience any of the following symptoms, which may be part of an allergic reaction: swelling of face, mouth, tongue, or throat, trouble breathing
Serious side effects are very rare and the common side effects are not bad. The injection site reaction is like the small bruise one gets following getting blood drawn in your arm for lab studies.
What are the benefits from using Emgality? In two 6-month studies of adults who had 4-14 migraine days per month Emgality cut migraine days in half for about 60% of people vs. about 39% for placebo.
In a 3-month study of adults with 15+ headache days per month, 28% had their monthly migraine days cut in half or more with Emgality compared to 15% who took placebo.
A migraine-free month is possible. This happened in 12% on average in one study and 16% in another vs. 6% who took placebo. In a 3-month study, the number of adults with 15+ headache days per month who were migraine-free in an average month did not differ between Emgality and placebo.
Emgality was specifically developed for migraine. Historically, many migraine preventive medicines were designed for other conditions. Emgality was specifically developed to bind to CGRP, a substance in the brain that may play a key role in migraine and block its binding to the receptor.
“The most exciting thing about these drugs is not the FDA-required endpoints,” says Alan Rapoport, MD, a clinical professor of neurology at UCLA’s David Geffen Medical School who has been a leader in headache research and treatment.
The value of the CGRP class is its potential to significantly reduce migraines for a reasonable number of patients. He says about 30% of patients may see 75% reduction, and between 15% and 20% may see a 100% reduction.
These are astounding results for migraine therapy and much better than the results of any previously used migraine preventive drug.
Emgality rock on
What are the results of previous migraine preventive drugs? The standard migraine preventive drugs are amitriptyline, topiramate, valproic acid, and the beta blockers-propanalol and atenolol.
All of these drugs work indirectly on the migraine process, while Emgality, blocking CGRP, acts directly in the migraine process. The older preventive drugs could reduce migraines about 30 % in a month and had a lot of side effects that resulted in poor compliance so that many patients just quit taking them.
Amitriptyline is an older antidepressant active at a dose of 100-125 mg and with reliable side effects of weight gain, dryness or mouth and constipation. Neurologists use dose of 10-20 mg with reduced, but still active side effects. However, it is the only migraine preventive drug, including the CGRP blockers, that helps with sleep and many migraine patients don’t sleep that well.
Topiramate is used at doses of 100-200 mg and also has predictable side effects of tingling lips and fingers, “cola tastes flat,” and trouble with cognition and word finding. Despairingly patients called it “dopamax.” Originally doses every 12 hours, long acting, once a day topiramate is available as Trokendi XR and Quedexy XR. It was originally used to treat epilepsy and then found to help with migraine.
Depakote ER or Valproic acid is another anticonvulsant drug later found to help migraine that has a complete contraindication for fertile women. It may cause malformed or “teratogenic” defects in babies which is unfortunate because migraine prevalence peaks at age 42 where 25 % of women get migraine and they are still having menstrual cycles and can’t take Depakote.
The betablockers propanalol and atenolol prevent the vasodilation that occurs during migraine, but they can also slow the pulse. They may cause a “tired syndrome” which makes them difficult to use although propranolol had the first FDA indication for a preventive migraine drug in 1974.
Previous oral migraine preventive drugs.
Special cardiovascular and cerebrovascular lack of risk. Migraine, especially migraine with aura, has an increased risk of cardiovascular and cerebrovascular risk. The main acute therapy drugs, the triptans, are contraindicated with cardiovascular and cerebrovascular disease because of a suspected increase of vasoconstriction with resultant heart attack or stroke.
However, an increasing number of studies show that vasodilation is not significant in migraine and that the new CGRP drugs are safe. The CGRP drugs are new and there are cries from some headache experts that more research should be done to further certify that CGRP drugs don’t relate to cardiovascular or cerebrovascular disease. Currently the FDA says that Emgality can be used in patients with cerebrovascular disease risk.
Emgality for Cluster headache prevention.
Cluster Classification. Cluster headaches are the most severe headache known to man. They are called “suicide headaches” for good reason. It is interesting that when the first American classification of headache was released in the JAMA in 1962 it had migraine as the main classification of headache and underneath the name migraine were listed different types of migraine such as common migraine, classical migraine, hemiplegic migraine, and at the bottom—cluster headache.
That is, cluster headache was originally listed as a type of migraine, but that was long ago and now the International Classification of Headache Disorder, first published in 1988, and now called ICHD III, has cluster headache as separate from migraine and called a Trigeminal Autonomic Cephalgia.
Does Emgality Work For Cluster Headaches?
The FDA has approved Emgality as the first-ever treatment for episodic cluster headache in adults that reduces the frequency of attacks. Emgality (galcanezumab-gnlm), is an injection medication originally approved for preventing migraine in adults in September 2018.
This approval comes after a clinical trial comparing Emgality to placebo (N=106) showed that patients taking Emgality experienced 8.7 fewer cluster headache attacks than patients taking placebo after 3 weeks. The most commonly reported adverse reaction during the trial was injection site reactions.
“Emgality provides patients with the first FDA-approved drug that reduces the frequency of attacks of episodic cluster headache, an extremely painful and often debilitating condition,” said Eric Bastings, MD, deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research.
Related questions.
Are there similarities and differences between migraine and cluster headache?
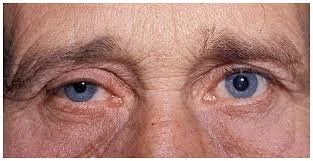
There are similarities and differences between migraine and cluster headache. Except for rare findings of one-sided eyelid drooping (ptosis) and one-sided pupillary smallness (miosis) found only in older patients who have had cluster headache for years and called Horner syndrome, both migraine and cluster headache have a normal neurologic exam.
Right sided lid droop and pupillary smallness
1.Normal MRI scan. Both migraine and cluster headache have normal cerebral MRI scans, except that 30-40% of migraine patients may have “microvascular T2 lesions” or “migraine freckles” which are benign and currently are thought to mean nothing serious.
2.Clinical occurrence. Episodic migraine occurs usually only several times a week and has symptoms of headache occurring on one side of the head in 80% of patients. However, migraine may switch sides, like from the right to the left side, although many patients will say that it “usually” just comes on one side.
Cluster headaches come without warning and reach a peak within 2-15 minutes. This is different from a typical migraine without aura attack which may take half an hour to several hours between onset and peak of headache pain. Cluster headaches are very severe, quick onset, one-sided headaches which consist of pain around the eye, temple, or cheek. Cluster headaches may track the clock, coming at the same time every day. This periodicity is a key feature of cluster headache; the attacks of pain recur at the same hour each day for the duration of the cluster bout.
Clock in my head
The attacks characteristically occur one to two hours after going to sleep in half of patients. An attack at this time corresponds with dreaming and REM stage sleep. Some patients have several attacks at night consistently interrupting sleep. Some patients get daytime attacks associated with napping or relaxation. Seventy-five percent of attacks occur between 9 p.m. and 10 a.m.
The duration of the attacks ranges from 15 to 180 minutes, with most cases lasting about 30 minutes to 2 hours with a mean of 45 minutes. The attacks may occur 1 to 8 times a day and the patient is pain free between attacks.
Attacks--the cluster period
The headaches come in time periods called “clusters” which usually last 6-12 weeks. The term “cluster” here means that the headaches cluster together in time, much like grapes cluster together on the vine.
Thus, the patient may state that he had 6 weeks of headaches in March and April of 2017, but then the headaches completely stopped. Following this there were no headaches in all of 2018, but they started again in March of 2019 and brought the patient to the doctor.
Although the usual cluster period is 1 ½ to 3 months, the average cluster time is 6 weeks. Ten percent of patients develop chronic cluster type headaches all year round and, in these patients, the “cluster” term is meaningless.
Also, cluster patients may cycle between intermittent or the typical "cluster" pattern and chronic daily cluster headaches. Chronic cluster patients may evolve into the episodic form without treatment.
Although some cluster periods occur in the spring and fall, other researchers have found cluster cycles in February and June that seem to occur at the time of increase in daylight hours. Cluster attacks occur 7 to 10 days before the longest and shortest days in the year, suggesting that the pineal gland located in the center of the brain may be involved.
3.Headache location. Cluster headaches are strictly one-sided temporal or supratemporal located pain. Only 15% of patients will switch sides for the duration of a bout. Very rarely the pain will switch sides during the cluster period.
Migraine may occur on one side in the temple, forehead, behind the eye, in the back of the head on one side, or in the cheek located trigeminal maxillary “sinus” on one or both sides of the face. Migraines starting in the back of the head on one side may move to behind the eye on the same side later during the headache period.
4.Episodes. Episodic migraine may come once or twice a week and is defined as lasting 4-72 hours. Chronic migraine, usually caused by medication overuse, is defined as having 15 headaches a month, 8 of which have migraine features.
Episodic cluster headache comes in “clusters” usually in the spring or in the fall lasting about 6 weeks. During the 6-week cluster time, headaches may come up to 8 times a day and last 15-180 minutes. Ten percent of cluster headache patients may have chronic cluster headache, which is continuous attacks with no remission.
5.Response to Triptans. Both migraine and cluster headache respond to acute triptan therapy. Cluster headaches come on so quickly and produce onset to peak pain in 2-5 minutes. Pain onset for migraine is usually longer--30-60 minutes. Because of their quick onset only large dose, fast onset triptan therapy will work. Suggested drugs are 6 mg subcutaneous sumatriptan, 25 mg nasal spray sumatriptan, or 5 mg nasal spray zomatriptan. Six mg subcutaneous sumatriptan is generally considered the best treatment.
Sumatriptan doses
Nasal spray sumatriptan and zomatriptan treatment onset is as fast as sumatriptan sc injectable—10 minutes. However, nasal spray sumatriptan gives a low level of medication in the brain of 10 mg while 6 mg sc sumatriptan gives a level of 100 mg. Injectable 6 mg sumatriptan is the most effective acute treatment for migraine and for cluster headache.
6.There is poor insurance coverage for sc sumatriptan for acute therapy of Cluster Headache. Sumatriptan is the best drug for treating cluster headaches. Glaxo, the pharmaceutical company that brought out sumatriptan only got an FDA indication for treating migraine, but never for cluster headache and it’s been 29 years (1991-2020) and no indication yet. It’s not coming.
Therefore, since medical insurance companies only approve and pay for drugs that have an FDA indication for use, United States medical insurance companies don’t adequately cover cluster headache. They will usually allow 9 of the 6mg sc shots of sumatriptan, but that’s it and not enough to cover a neurological condition that may occur 8 times a day every day for 6 weeks.
There are articles warning that medication overuse headache may occur with cluster headache, but it must be rare unless the patient is mistakenly taking opioids or butalbital for treatment.
7.Response to oxygen. Cluster headache usually responds quickly to nasal oxygen delivered via a scuba tank oxygen cylinder, tubing, and nasal prongs or a mask. This is great since oxygen is, well, oxygen and it’s not toxic or expensive, and it will usually stop a cluster attack in minutes. Also, there is some older data on success with nasal oxygen for migraine, although it usually requires a longer duration of therapy, like an hour.
Nasal Oxygen
8.Indications for Emgality (galcanzumag-gnlm). Emgality is a prescription medicine used in adults for:
The preventive treatment of migraine. The medicine (120 mg) comes in a prefilled pen or syringe and is taken once a month.
The treatment of episodic cluster headache. The medicine (300 mg) comes in three (100 mg) prefilled syringes, which are taken one after the other at the start of a cluster period and then every month until the end of the cluster period.
9.Warnings and side effects of Emgality for Cluster Headache.
Do not use Emgality if you are allergic to galcanezumab-gnlm or any of the ingredients in Emgality.
Emgality may cause allergic reactions, such as itching, rash, hives, and trouble breathing. Allergic reactions can happen days after using Emgality. Call your healthcare provider or get emergency medical help right away if you have any of the following symptoms, which may be part of an allergic reaction: swelling of your face, mouth, tongue, or throat, or trouble breathing.
10.Use with other drugs. Emgality is one of three new CGRP FDA approved drugs for treating migraine, and the only of the group indicated for treating cluster headache. Emgality blocks the effect of CGRP, one of the neurotoxins released in the brain with migraine and cluster headache.
These CGRP antibody drugs have no effect with any other known drug and can be used liberally and safely with any other drug. For instance penicillin has 10 or more drugs that cannot be used when the patient is on penicillin, but the CGRP drugs can be used with any drug.
11. Emgality common side effects.
The most common side effects of Emgality are injection site reactions. This is from the subcutaneous injection with a small needle and is like the small bruise one may get from having blood for lab tests drawn from the arm. Diabetics give sc shots four times a day for life.
10.Migraine without aura symptoms. To have an accurate diagnosis of migraine there should be:
A. Five similar attacks with symptoms as follow below.
B. The migraine attacks last 4-72 hours.
C. The attacks should have 2 out of the following 4 symptoms:
One sided pain
Throbbing or pulsating pain
Pain that is moderate or severe, on a 1-10 pain scale migraine is over 5-up to 10
Migraine pain is disabling so the patient wants to be still or lie down and sleep; thus, an attack of migraine may limit social activity or work.
D. The attacks should have 2 of the following 4 symptoms:
Nausea
Vomiting
Sensitivity to light-photophobia
Sensitivity to sound-sonophobia.
11.Cluster symptoms.
ICHD-3 Beta Definition of Cluster Headache
A. At least 5 attacks fulfilling B-D below.
B. Severe unilateral orbital, supraorbital, and/or temporal pain lasting 15 to 180 min.
C. Attack is associated with at least one of the following signs on the side of pain:
1. Conjunctival injection
2. Lacrimation
3. Nasal congestion
4. Rhinorrhea
5. Forehead and facial sweating
6. Miosis
7. Ptosis
8. Eyelid edema
D. Frequency: from one every other day to eight per day
E. At least one of the following:
1. History, physical, and neurological examinations do not suggest disorders in groups 5-11 of IHS classification.
2. History and/or physical and/or neurological examinations do suggest other disorder, but it is ruled out by appropriate investigations.
3. Such disorder is present, but tension-type headache does not occur for the first time in close temporal relation to the disorder.
Clinical features
During the cluster headache in addition to pain the patient may experience symptoms on one side of the face around the eye, upper cheek, or temple. These symptoms may be:
drooping of the upper eyelid (ptosis)
smallness of one pupil (miosis)
sweating above the eye
redness of the eye (conjunctival injection)
tearing of one eye (lacrimation)
nasal congestion or drainage of clear fluid (rhinorrhea)
These “sinus” type symptoms many times bring the patient to the general doctor or ear nose throat surgeon with a self-made diagnosis of “sinus headache” with resultant treatment with repeat doses of various antibiotics or occasional sinus surgery.
Pain description
The pain begins quickly, without warning. It is excruciating in intensity and explosive in quality. Rarely the pain is pulsatile. Patients describe the headaches as “killer” headache or “suicide headache.” The pain may be “boring, stabbing, burning, like a knife, like a hot poker in the eye” and sometimes “pulsating, throbbing, squeezing, or aching.” At the end of the attack the symptoms resolve in 1-2 minutes
12.Warning for pregnancy and breastfeeding.
None of the CGRP drugs, including Emgality, are indicated during pregnancy and while breastfeeding. None of the CGRP drugs are approved for children or persons under 18 years of age.
13. What to do for a missed dose. If a dose of Emgality is missed, then the dose should be given as soon as the patient is aware of it and able. Then that date of injection is used as the new marker to count one month to the next dose. Put dosing of medication in your cell phone with an alarm marking the date and time. Also tell your family, friends, or sometimes your co-workers your dosing schedule so that they may help you not forget.
14. Confusion between Cluster Headache and Chronic Migraine due to medication overuse headache. Patients and doctors get these two headache entities mixed up all the time and I hear it in the office. The patient will have a headache pattern with migraine but not cluster features. The “cluster” comment is added because the headaches are so frequent, often times daily for months or years.
Cluster headache usually occurs in men, type A, hard driven types. I once saw a patient with cluster headache who had 4 beepers, remember beepers for work back in the seventies? He had a beeper for his business, one for being the chief of the police department in the small Texas town where he worked, another for being chief of the fire department, and another for his mother, who was sick and bedfast.
But these social/psychiatric features don’t separate cluster from migraine. Cluster headache comes the same time every day, multiple attacks every day and they lock into time, like 02:00 AM or at 09:00, every day. And cluster headaches are quick onset to peak, extremely severe headaches that last an average time of 45 minutes, while migraines onset to peak is lots longer and an episode of migraine can last 4-72 hours.
Chronic migraine due to medication overuse headache is usually a “wake up” headache that gets better after the patient takes his butalbital, only to return hours later as another severe headache. Medication overuse headache used to be called “rebound headache.” The headache with medication overuse headache doesn’t really go away, it may persist as pain in the neck of back of head, whereas between attacks cluster headache really goes away.
The big features of cluster headache are the autonomic features: forehead sweating, one sided lid drooping, a small pupil noted by the very observant patient, or spouse, redness of the conjunctiva, lacrimation, usually just on the one cluster side, and nasal congestion. Migraine may rarely have some of these features, but migraine has strong throbbing, nausea and vomiting, and sensitivity to light and sound.
The activity of the patient also will give it away. Cluster headache patients get up and pace around the room. They are hyper, in extreme pain. Sometimes they’ll push something sharp like a paper clip in the flesh of their head where it hurts so badly.
Migraine patients go down. They go to bed, they try to go to sleep, because sleep may reset their brain and treat the migraine.
What about use of the new CGRP antibody drugs for pregnancy or lactation? There is no current indication for the use of a CGRP antibody drug like Emgality during pregnancy or while breastfeeding. The FDA statement regarding CGRP antibody drugs is:
Pregnancy-caution is advised during pregnancy. No human data is available, no known risk of fetal harm based on animal data at 20 times recommended human dose.
Lactation–caution is advised for breast-feeding. No human data available to assess risk of infant harm or effects on milk production.
Check out my Big Book on Migraine Here
Emgality should not be taken during pregnancy or breastfeeding.
Emgality !
This site is owned and operated by Internet School LLC, a limited liability company headquartered in Dallas, Texas, USA. Internet School LLC is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.com. Although this site provides information about various medical conditions, the reader is directed to his own treating physician for medical treatment.
All the best.
Follow me at: www.doctormigraine.com, Pinterest, Amazon books, Podcasts, and YouTube.
Britt Talley Daniel MD