Does Aimovig Treat Migraines?
The migraine process generates three neurochemicals, Neurokinan A, Substance P, and CGRP. These neurochemicals are released by the ganglia of the trigeminal nerve and cerebral arteries. They cause the cerebral arteries to dilate and inflame the trigeminal nerve, the cerebral arteries, and the thalamus. The thalamus is the “pain center” of the brain. No other medical problem inflames the thalamus like migraine does. Triptans, if taken early in the migraine process, 20-40 minutes after headache onset, block the release of these inflammatory neurochemicals and are the best drugs currently for acute therapy for migraine.
This is an article by Britt Talley Daniel MD, member of the American Academy of Neurology, the American Headache Society, migraine textbook author, and blogger.
It is known that these neurochemicals are released by the migraine process by experimental research wherein a patient was given a drug to start the migraine and blood was collected from their jugular vein by a needle in their neck as the neurochemicals were on their way from the brain, to the liver to be metabolized, and then to leave the body in the toilet. After the migraine started there was a marked increase in the three neurochemicals listed above. Without treatment with a triptan early, it takes 72 hours for the neurochemicals to leave the body. Some migraine patients with Chronic Migraine (at least 15 headache days/month, 8 of which have migraine features) get this from overtreating with triptans, caffeine, NSAIDs, opioid narcotics or butalbital and in this scenario every time they drink a cup of coffee or take 2 more Tylenols, they get 3 more days of the neurochemicals and the headache and the neurochemicals keep coming like gasoline on a wood fire.
The Migraine Timing Cycle
Aimovig is a new CGRP blocking preventive drug for Migraine. CGRP is a neurochemical which is released during in the migraine process and blocking it significantly improves migraine attacks. Aimovig has few side effects, can be taken safely with all other drugs, and is given by a subcutaneous injection monthly. Aimovig is the first CGRP blocking drug and the first to enter into the pathophysiological Migraine process to provide relief from Migraine attacks.
Read my Mini Book on Migraine Here.
Does Aimovig interact with other drugs? Most modern drugs are made from the dirt or plants in the world and then are processed by drug companies and used to treat a specific illness. For instance, penicillin is a mold that accidentally dropped on an agar plate Fleming had put on his window ledge. Penicillin was then later manufactured and sold to treat bacterial illness. Penicillin has 10 different drug contraindications. This means that penicillin should not be used with 10 different drugs which then may harm the patient.
Aimovig has no drug interactions. Aimovig can be taken with any other drug. It is made by DNA polymerization and then injected in the body. It is not known to affect the menstrual cycle.
No interaction with any other drug.
What are the side effects from taking Aimovig? Serious side effects are hypersensitivity reaction; common side effects are injection site reaction, constipation, and muscle cramps or spasms. Serious side effects are very rare and the common side effects are not bad. Constipation may occur in 3% but it improves with time. The injection site reaction is like the small bruise one gets following getting blood drawn in your arm for lab studies.
Small bruise at site of Aimovig subcutaneous injection
What are the benefits from using Aimovig? Half of patients get 50 % reduction in migraine headaches. Another 25 % of patients get 75 % reduction in migraine and another 25 % of patients, the so-called “super responders” get 100 % reduction of migraine headaches.
“The most exciting thing about these drugs is not the FDA-required endpoints,” says Alan Rapoport, MD, a clinical professor of neurology at UCLA’s David Geffen Medical School who has been a leader in headache research and treatment. The value of the CGRP class is its potential to significantly reduce migraines for a reasonable number of patients. He says about 30% of patients may see 75% reduction, and between 15% and 20% may see a 100% reduction.
Rock on Aimovig
These are astounding results for migraine therapy and much better than the results of any previously used migraine preventive drug.
What are the results of previous migraine drugs? The standard migraine preventive drugs are amitriptyline, topiramate, valproic acid, and the beta blockers-propanalol and atenolol.
All of these drugs work indirectly on the migraine process, while Aimovig, blocking CGRP, acts directly in the migraine process. These older preventive drugs could reduce migraines bout 30 % in a month and had a lot of side effects that resulted in poor compliance so that many patients just quit taking them.
Amitriptyline is an older antidepressant active at a dose of 100-125 mg and with reliable side effects of weight gain, dryness of mouth. and constipation. Neurologists use a dose of 10-20 mg with reduced, but still active side effects. However, it is the only migraine preventive drug, including the CGRP blockers, that helps with sleep and many migraine patients don’t sleep that well.
Topiramate is used at doses of 100-200 mg and also has predictable side effects of tingling lips and fingers, “cola tastes flat,” and trouble with cognition and word finding. Despairingly patients called it “dopamax.” Originally dosed every 12 hours, long acting, once a day topiramate is available as Trokendi XR and Quedexy XR. It was originally used to treat epilepsy and then found to help with migraine.
Depakote ER or Valproic acid is another anticonvulsant drug later found to help migraine that has a complete contraindication for fertile women. It may cause malformed or “teratogenic” defects in babies which is unfortunate because migraine prevalence peaks at age 42 where 25 % of women get migraine and they are still having menstrual cycles and can’t take Depakote.
The betablockers propanalol and atenolol prevent the vasodilation that occurs during migraine, but they can also slow the pulse. They may cause a “tired syndrome” which makes them difficult to use although propanalol had the first FDA indication for a preventive migraine drug in 1974.
The CGRP drugs change migraine medication from combating cerebrovascular changes and vasodilation. An increasing number of studies show that vasodilation is not significant in migraines, and clinicians and some informed patients are excited that a new pathway is being pursued that will get at the excruciating pain migraines can cause.
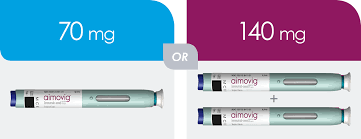
Is 70 mg subcutaneously the only dose of Aimovig? No, the FDA approved the 70 and 140 mg doses and both are available for migraine patients. In general doctors start with the 70 mg dose for 3-6 months and then move up to the 140 mg dose.
Related questions.
What kind of injector is used for Aimovig?
Aimovig comes in a 70 mg SureClick single dose autoinjector or in a 140 mg dose.
Aimovig autoinjector
How does the autoinjector work?
The autoinjector has a hidden needle.
It should be stored refrigerated at 36-46 degrees F in a refrigerator in the original carton to protect it from light until it is used.
If the autoinjector is removed from the refrigerator, it should be kept at 77 degrees F in the original carton. It should be used within 7 days or thrown away.
Leave the autoinjector at room temperature for 30 minutes before injecting.
How is the injection given?
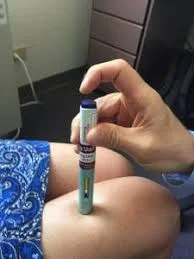
Clean the thigh or abdomen with an alcohol sponge. Place the autoinjector on the skin. Push the button at the end or the injector and a click will sound, and the medication will be injected subcutaneously in 15 seconds. Another click will sound. Release the injector from the skin and dispose of it using a biologic needle safety site.
Refrigeration
Aimovig should be kept refrigerated until it given to the patient. Product information regarding Aimovig states: “After removing AIMOVIG from the refrigerator, it can be stored at room temperature between 68°F to 77°F (20°C to 25°C) for up to 7 days. Throw away AIMOVIG that has been left at room temperature for more than 7 days. Do not freeze.
Check out my Big Book on Migraine Here.
This site is owned and operated by Internet School LLC, a limited liability company headquartered in Dallas, Texas, USA. Internet School LLC is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.com. Although this site provides information about various medical conditions, the reader is directed to his own treating physician for medical treatment.
All the best.
Follow me at: www.doctormigraine.com, Pinterest, Amazon books, Podbean, and YouTube.
Britt Talley Daniel MD
Giving Aimovig autoinjector subcutaneous shot.